Measuring the temperature in the room
The task:
Measure the ambient (air) temperature in the room and the temperature of some bodies in the room.
The tools:
- uLAB BOX
- uLAB SENSOR TE01 (temperature sensor)
- beaker with water 100 ml
Arrangement:

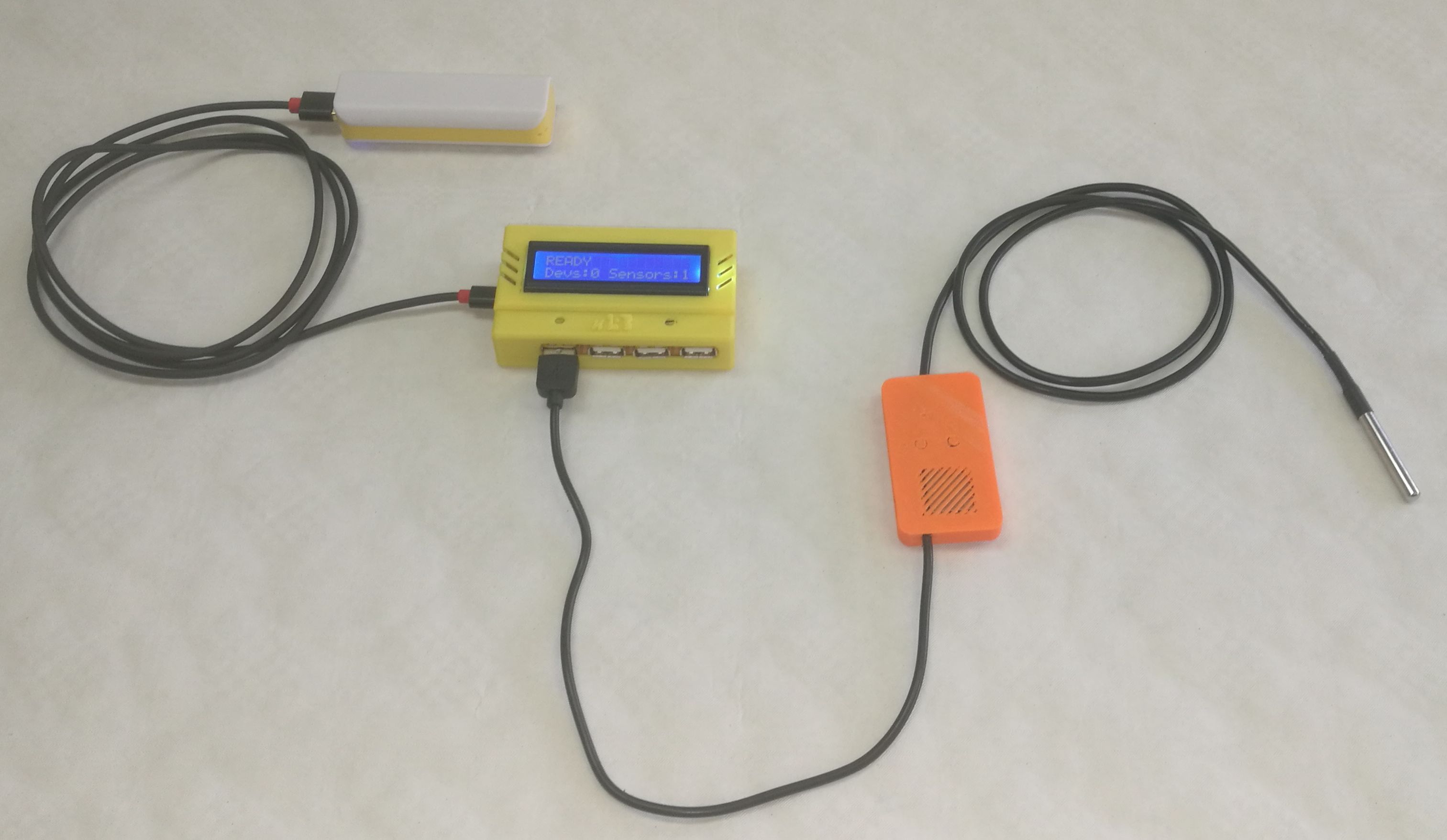
Temperature measurement – a) set-up of the experiment, b) picture of the experiment
Procedure:
1. Pour water from the tap into the prepared beaker and measure its temperature by immersing the sensor in the liquid.
2. Also measure the temperature in different places of the room, focusing mainly on places near doors, windows, various openings, etc.
3. The measurement is carried out using either the time measurement module or the XY dependency measurement module
- in the case of time measurement (Time based measurement), we read the temperature values from the analog scale or digital indicators, while recording the measured temperatures in the prepared table
- in the case of using XY dependence, we turn on the Manual channel (by clicking on it and renaming the sensor to “place”, for example) and choose the axes (Man x-axis, temperature y-axis); we can perform the measurement in two ways:
- after turning on the automatic increment of the measurement number (clicking Man autoincrement), the system will automatically increase the measurement number after recording the measured value
- the value of the Man channel will be entered manually before each measurement (e.g. “at the window”); the Man autoincrement box must not be checked in that case
4. Use the mouse or the spacebar to record the current measured temperature in the selected location.
5. It is advisable to take a photo of the laboratory or room and mark the measurement locations with the measured temperature (next fig.)
6. We can measure the water temperature in the beaker in two ways:
- measure the temperature of the water in the beaker during 30 minutes
- measure the water temperature continuously thanks to the time measurement module, in which we set the reading interval to 5 s and the duration of the measurement to 30 minutes
Security:
Attention must be increased only in the case of using bodies with a temperature higher than 60 °C, or with a temperature lower than -10 °C.
Processing:
The temperature in selected places of the room was read only at the moment when the temperature balance between the thermometer and the surroundings was reached, i.e. when the temperature stopped changing.

Measuring the temperature in the room
Assuming that there is no heat source near the window (for example, a radiator for heating), it can be concluded that the outside temperature is higher than the temperature even in a non-air-conditioned room, and therefore it is highly probable that the measurement was carried out in the summer. This conclusion is also supported by the fact that the temperature decreases with increasing distance from the window (a so-called temperature gradient is observed). Based on the measurement, a table was created:
| place | temperature | thermodynamic temperature (calc.) | |
| [-] | [°C] | [K] | |
| 1 | air in the beaker | 24.45 | 297.60 |
| 2 | water in the beaker | 21.31 | 294.46 |
| 3 | window | 26.26 | 299.41 |
| 4 | door | 24.57 | 297.72 |
| 5 | screen | 24.28 | 297.43 |
| 6 | water in the beaker after 30min | 22.12 | 295.27 |
| 7 | air in the beaker after 30min | 24.35 | 297.50 |
The time dependence of the temperature of the water in the beaker was measured in such a way that after the start of the measurement, the thermometer was immersed in the water in the beaker after 20 seconds. The slight increase in temperature before inserting the thermometer into the water (first Chart) can be explained by the presence of a human operator (with a body temperature of almost 37 °C). After this time, during the thermal exchange of the temperature sensor with the water in the beaker, the measured temperature drops rather sharply to a value that is close to the real temperature of the water in the beaker, and therefore also of the thermometer.
After about 100 seconds, it can be concluded that the process of heat exchange between the thermometer and the liquid is already completed, since the temperature practically does not change anymore (or its change is minimal). The slow increase in the temperature of the water in the beaker is caused by the gradual heat exchange between the beaker and the environment, which progress relatively slowly (in 30 minutes after the start of the measurement, the temperature balance still did not occur). As the heat exchange depends on the temperature difference, it can be assumed that the heat exchange will slow down, while the measured temperature should, assuming unchanged initial conditions, asymptotically approach the ambient temperature.
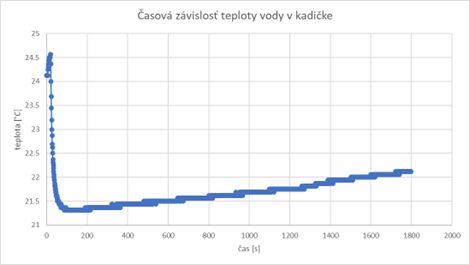
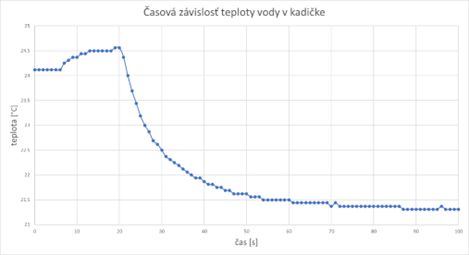
Questions:
- In which place is the maximum and minimum temperature (also consider the reasons)?
- How can the situation differ depending on the season?
- Why do the compared thermodynamic temperature data (calculated and measured) differ?
- What happens to the water in the beaker and what is the state of the system called at the beginning and at the end?
- Why did the ambient temperature not increase (or decrease only minimally)?
- What would happen next after the end of the measurement?
- What would happen if the temperature of the water in the beaker was higher than the temperature of the air?
Answers:
- Maximum and minimum temperature:
- the maximum temperature was measured in the window area, because the thermometer was located in direct sunlight
- the minimum temperature was measured in the left part of the image, near the entrance door to the laboratory (outside the image), where higher air circulation is assumed (in this case, it was air circulation from cooler areas of the building)
- Influence of the season on the measurement:
- it can be expected that in winter the outside temperature is lower than in the room, and due to the imperfection of the thermal insulation ability of the window, heat leakage through the window, and therefore the temperature near the window will be lower than in the middle of the room
- heating elements (radiators) are usually placed under the window in order to achieve the effect of air circulation (warm air has a lower density and therefore always flows upwards); if the thermometer is placed near the radiator, we can measure a higher temperature than in the middle of the room
- if the thermometer is placed around the door, it is difficult to predict its behavior, because there is not enough information about the temperature conditions in the space behind the door
- The difference between calculated and measured was because:
- the limited accuracy of the sensor as well as (slightly) different measurement locations
- the conditions between the two measurements may have changed slightly (we should be remember that the accuracy of the thermometer is at the level of 0.0625 °C), which can be influenced just by the presence of a person near the sensor
- Water in a beaker
- is heated in this case because its initial temperature was lower than the ambient temperature (24.26 °C)
- such a condition is called non-equilibrium, which causes the transfer of heat from a warmer body (the air and all bodies in the room) to a cooler body (the water in the beaker)
- heat transfer occurs until both temperatures are equal, in which case the system will be in equilibrium state
- the temperature of the water in the beaker has increased, but thermal equilibrium has not yet occurred at the time the measurement is finished, because the temperatures of the water and the air are not the same
- The temperature of the environment (air and all objects in the room) has practically not changed, because their weight compared to the weight of the water in the beaker is significantly higher (compare calorimetric equation Q=m.c.ΔT, ∑Qi =∑Qj)
- The temperatures would equalize after a certain time and the system would thus be in an equilibrium state, assuming thermal insulation of the system; in fact, it is very difficult to ensure the isolation of the system
- In that case, the water would transfer heat to the environment and the temperature of the water would gradually decrease until both temperatures became equal
